Technology
Longitudinal Assay Screening (LAS)
Conceptually similar to real-time PCR for proteins, LAS is based on the iterative flow of sample and labeled detection reagents across assays housed on fluidic cartridges. Multiple ‘time-resolved’ fluorescent measurements are taken of the formation of the sandwich between capture reagent, analyte of interest, and detection reagent (‘real-time ELISA’). The below figures illustrate the LAS process alongside the time-resolved data collection and processing steps.
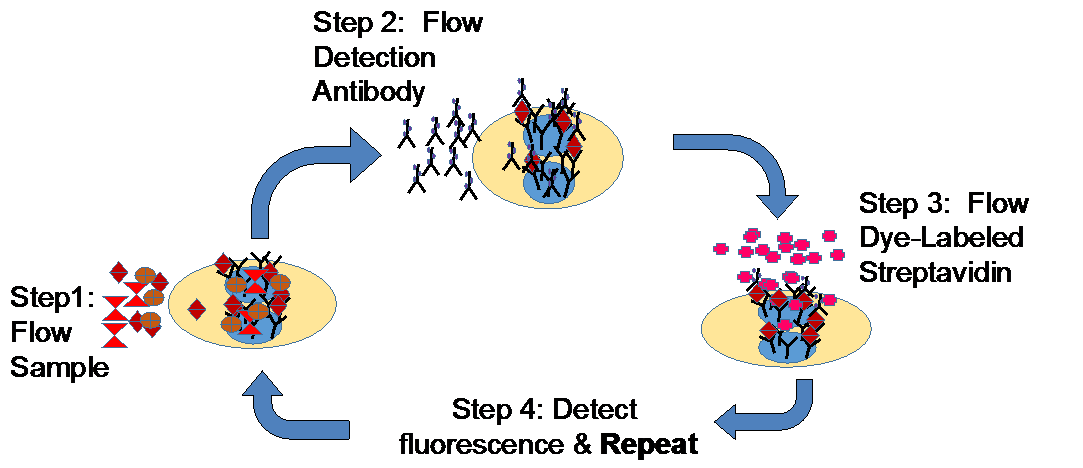
Schematic of LAS process
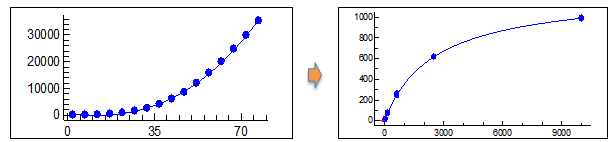
Illustration of the ‘time-resolved’ data generated through LAS. On the left had side you can see the fluorescent intensity (y-axis) build over time (x-axis). This time-course data is then converted into ‘rate’ data (right hand side) through the Bio-ID’s software.
Due to the time-resolved nature of the assay, the resulting data is a ‘rate of reaction’ instead of a simple final fluorescent reading. This is processed through proprietary analytics software, delivering users accurate data on the concentration of all proteins within the assay in real-time.
- LAS solves the ‘limited detection range’ problem as the novel rate based analysis enables the accurate quantitation of protein concentrations across over a 7log range in a single multiplex test. This eliminates the need for serial dilutions, making multiplexing faster, cheaper and helping preserve precious samples.
- LAS solves the ‘limited biological relevance’ problem as due to its large detection range, LAS allows users to run virtually any assay of interest in one test, enabling the development of truly biologically relevant multiplexes.
- LAS solves the ‘limited data accuracy’ problem by producing and analyzing real-time kinetic data on protein interactions (time-course and rate of reaction data instead of a single data point); delivering users with a greater depth of information on each assay so that a more accurate and reliable assessment of the assay results can be made.
LAS delivers a unique and transformative solution to the detection and measurement of multiple proteins for both clinical and R&D applications. Given its unique advantages, LAS technology is well positioned to become to proteomics what real-time PCR was to genomics.
The Bio-ID is the world’s first platform to integrate LAS technology. Details on the Bio-ID and associated demonstration data are included in the Bio-ID White Paper.
Inanovate’s technology is covered by 7 patents, protecting the LAS process, flow control methods, the Bio-ID cartridge, surface technology, and analysis methods. Inanovate also holds exclusive licenses to biomarkers for prostate and ovarian cancer presently being assessed using the Bio-ID.
